Research
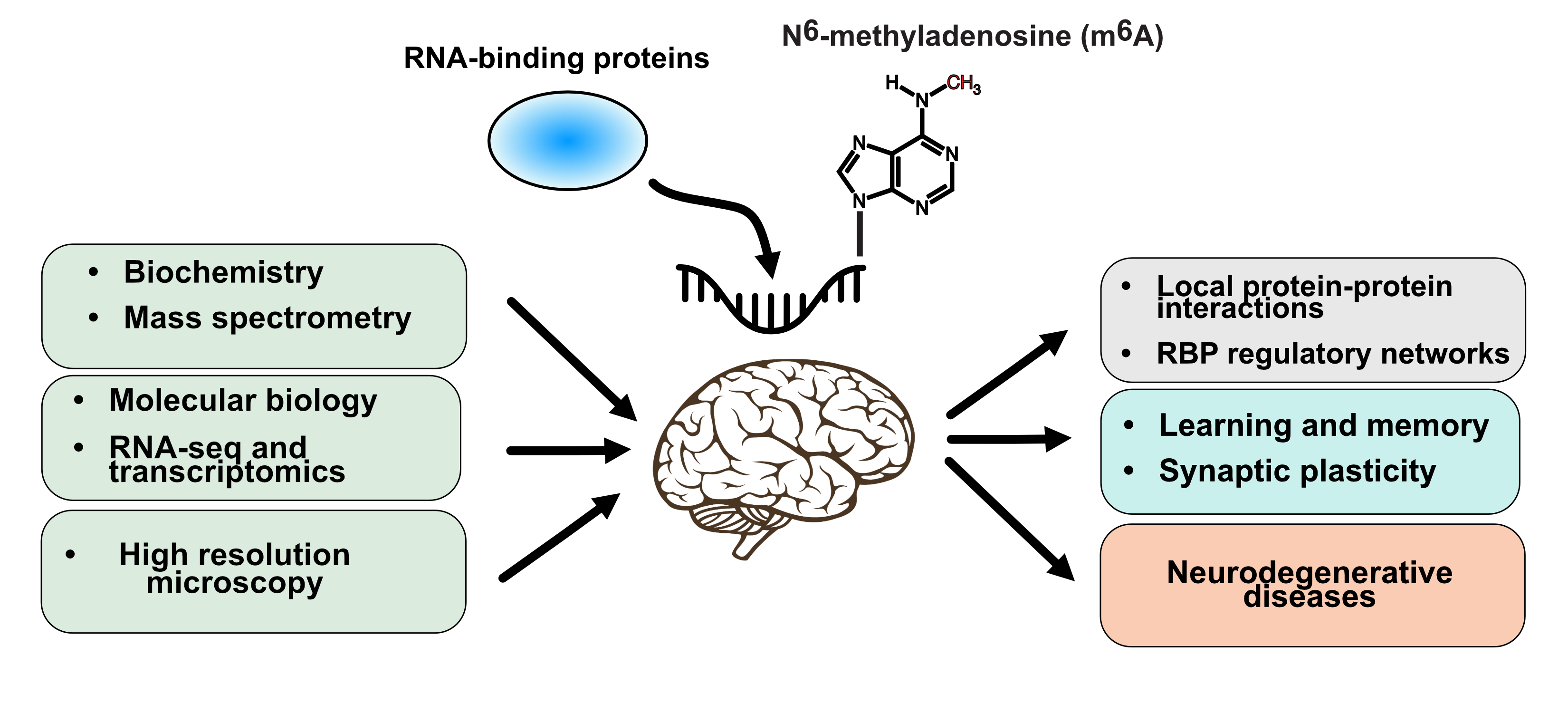
Our lab focuses on understanding the molecular mechanisms by which RNA modifications and RNA-binding proteins (RBPs) contribute to gene regulatory networks in neurons. We currently have two projects in the lab:
Characterize protein-protein and protein-RNA interaction networks at synapses.
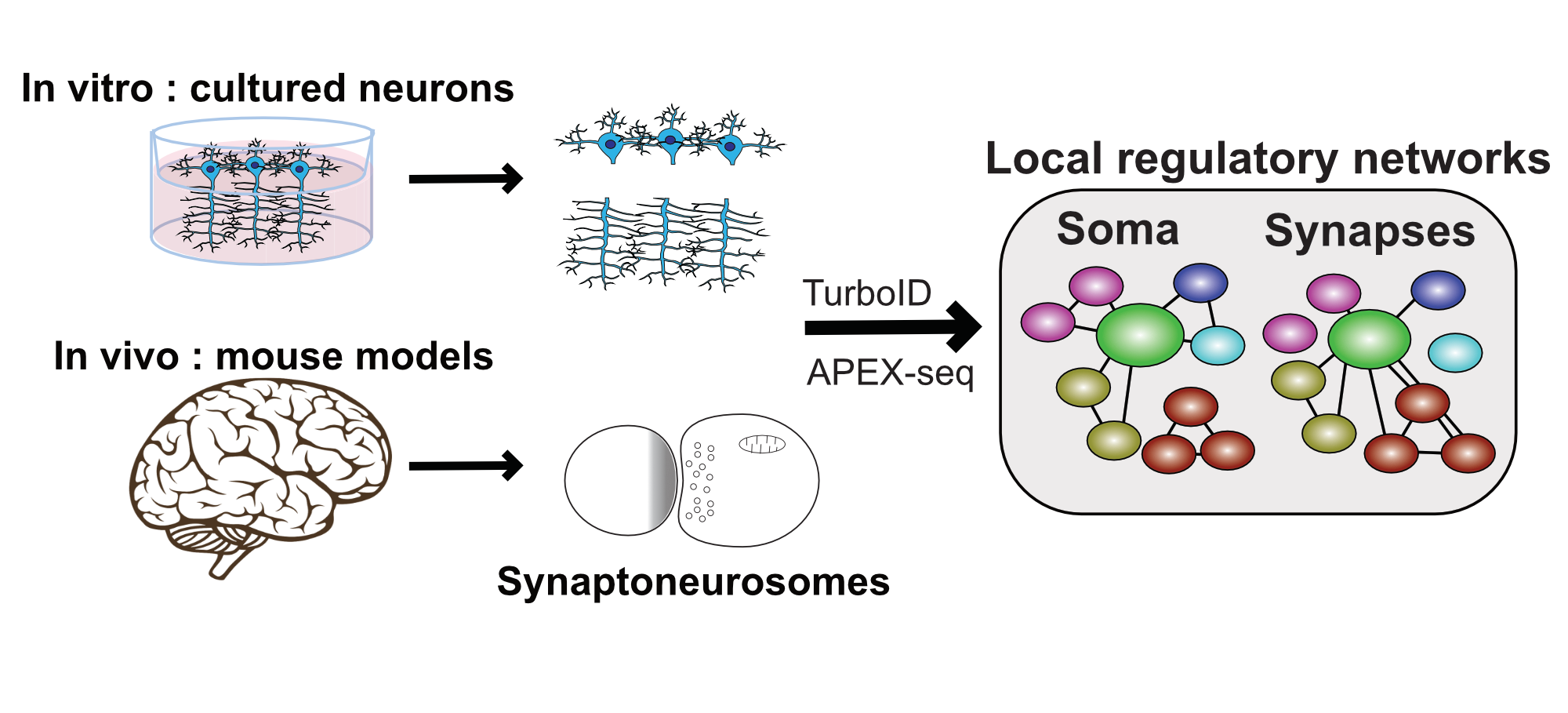
In neurons, the local translation of mRNAs is an essential process enabling rapid changes in local protein levels following synaptic transmission events, and which enables in memory formation. RBPs are central players in large regulatory networks which control mRNA transport, decay, and local translation. As such, the loss and mutation of RBPs are linked to a wide range of neurological disorders. To understand the function of these proteins on synaptic plasticity and memory formation, it is crucial to characterize the interaction networks localized at synapses. To this end, Mathieu Flamand’s team employs cutting-edge biochemical, proteomic, and cellular imaging techniques to characterize RBP interactions and function at synapses, using both cultured neurons and mouse models.
Understanding the disruption of m6A regulatory networks in neurodegenerative diseases.
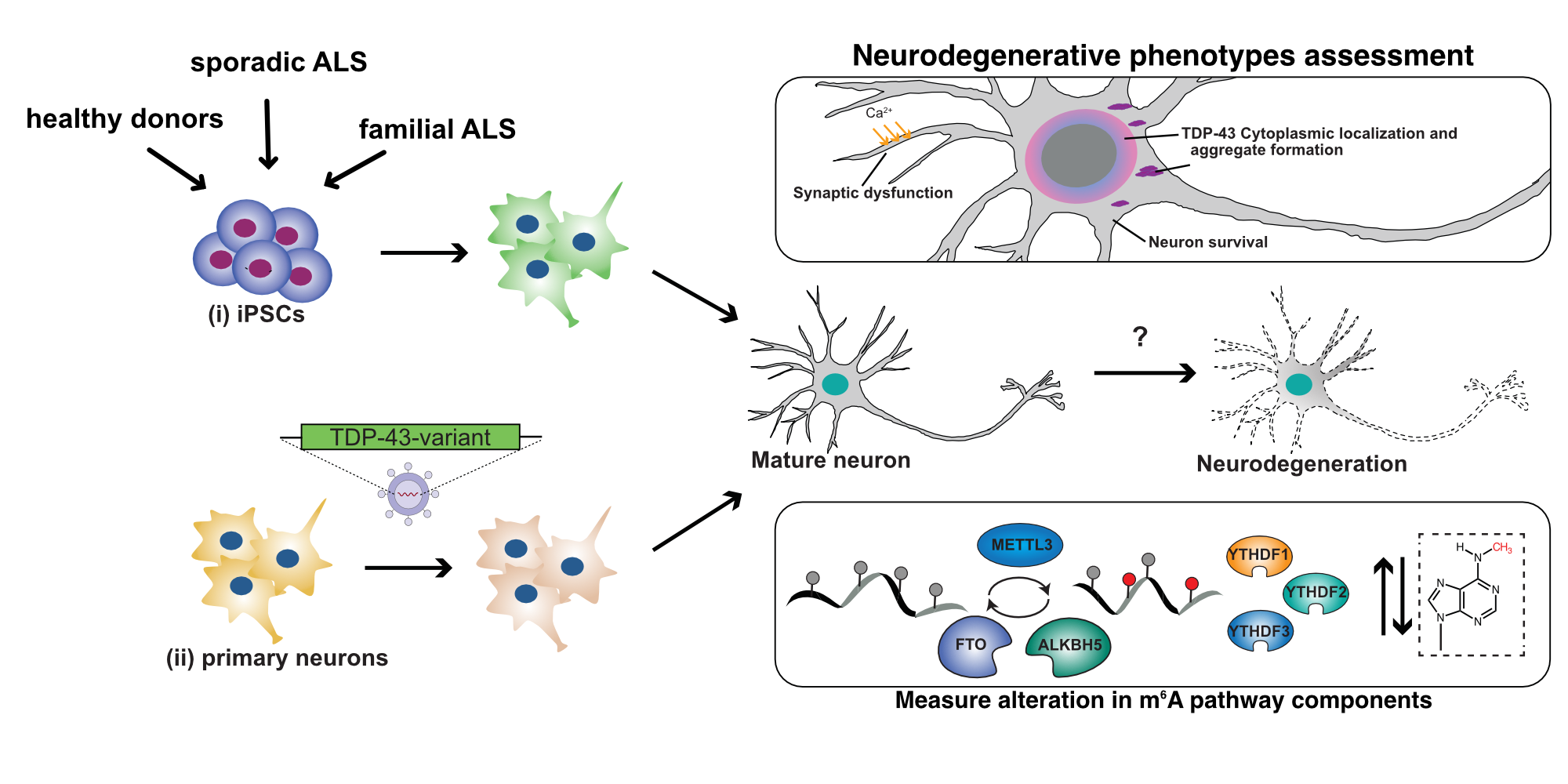
Increasing evidence indicates that m6A and m6A-readers are essential to synaptic function. Moreover, disturbances in m6A levels are linked to neurodegenerative disorders leading to memory impairment, including amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD). However, the molecular mechanisms by which they contribute to these diseases remain largely unknown. To better understand this problem, Mathieu Flamand’s team aims to understand the cooperative interactions between RBPs, and the alterations to m6A regulatory networks during these diseases. For this, his group uses a combination of molecular biology and genomics tools he developed to probe the targets and regulation of RBPs in cultured neurons, iPSC, and mouse models. These approaches will identify the critical roles of the m6A pathway in ALS and AD, identify new biomarkers, and provide information on the viability of therapeutically targeting of m6A.
For these projects, we are currently supported by: